Case History
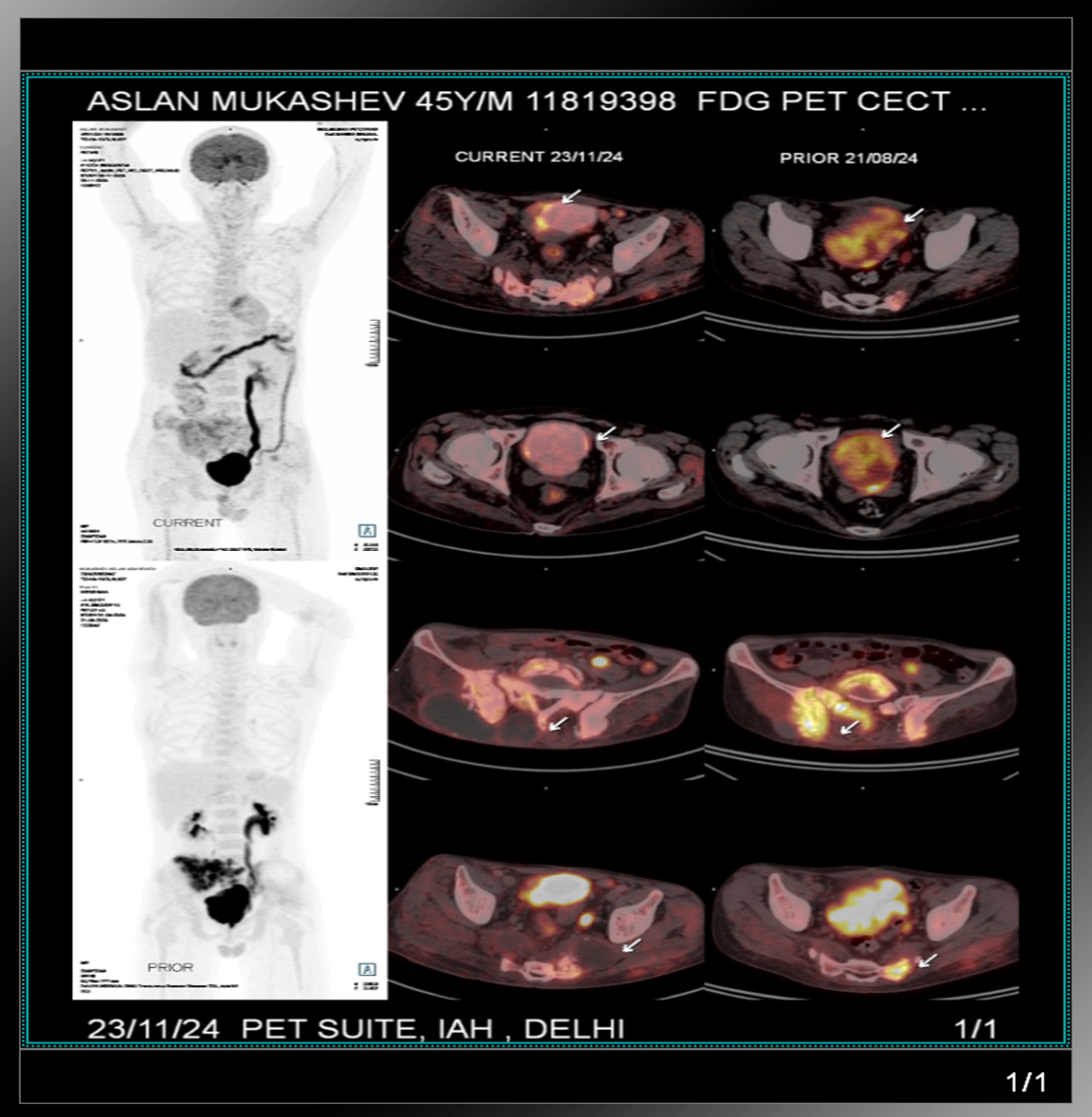
PET CT (21.08.2024) (Translated)
PET CT reviewed in India
PET CT CD review revealed extensive mets in right sacrum, ileum and other skeletal mets.
USG KUB on 17.09.2024
DTPA Renal Dynamic study on 18.09.2024
2D Echo on 18.09.2024
Cystoscopy with TURBT with Biopsy was done on 20.09.2024
Treatment protocol
Chemotherapy + ImmunotherapyReview of literature
One open-label, phase III randomized controlled trial (RCT) (Study EV-301) comparing enfortumab vedotin to standard salvage chemotherapy with docetaxel, paclitaxel, or vinflunine in adult patients with locally advanced or metastatic UC who had received a platinum-containing chemotherapy and who had experienced disease progression or relapse during or following treatment with PD-1 or PD-L1 inhibitors, demonstrated that treatment with enfortumab vedotin resulted in added clinical benefit with a statistically significantly prolonged overall survival (OS) (hazard ratio [HR] = 0.702; 95% confidence interval [CI], 0.556 to 0.886; P = 0.00142) and progression-free survival (PFS) (HR = 0.615; 95% CI, 0.505 to 0.748; P < 0.00001) compared to chemotherapy. The confirmed overall response rate (ORR) was also statistically significant in favour of enfortumab vedotin at 40.6% compared to 17.9% for chemotherapy (P < 0.001).
Evidence from Study EV-301 showed that enfortumab vedotin was associated with a significantly prolonged OS, with a median OS of 12.88 months for enfortumab vedotin compared to 8.97 months with chemotherapy (HR = 0.702; 95% CI, 0.556 to 0.886). Though statistically significant, pERC considered the results for OS moderate, which was a concern given that Study EV-301 was stopped early for efficacy based on an information fraction of 68.6%; thus, the moderately meaningful OS benefit seen might be an overestimation of the true benefit that could be conferred by enfortumab vedotin. Results for PFS and ORR were consistent with the primary end point. However, pERC noted that there are no treatments for patients that have failed platinum-based chemotherapy and experienced disease progression on PD-1 or PD-L1 inhibitors that has demonstrated improved survival; thus, the Committee considered the benefit of OS and PFS to be clinically meaningful.