Case Summary:
-
47 year female
-
Non smoker
-
Cough for 2 months
-
Diagnosed as metastatic carcinoma lung in 2014
-
Adenocarcinoma
-
NO ACTIONABLE MUTATION
Treatment
-
Pemetrexed and carboplatin was started (JMDBstudy)
-
PET/CT s/o PARTIAL RESPONSE
-
Pemetrexed maintenance ( PARAMOUNT STUDY)
-
Post 11 cycles patient progressed
-
DOCETAXEL ( TAX 317 and 320 study)
-
Post 3 cycles assessment was done , WB PET /CT scan s/o PARTIAL RESPONSE
-
Regular follow up - STABLE DISEASE in subsequent scans
-
Patient was doing well clinically
-
Post 50 cycles of DOCETAXEL - Progressed with brain metastasis (June 2018)
-
WBRT and RT to right lung lesion
-
Atezolizumab 1200 mg every 3 weeks for 7 cycles (June 2018 to Nov 2018)
-
WB PET/CT scan -PROGRESSIVE DISEASE (Nov 2018)
-
Started on gemcitabine and carboplatin
-
Reassessed after 2 cycles PET/CT scan s/o STABLE DISEASE
-
So gemcitabine and carboplatin continued for total 6 cycles
-
Post 6 cycles PET/CT s/o STABLE DISEASE (June 2019)
-
Meanwhile pleurodhesis was done in view of persistent pleural effusion
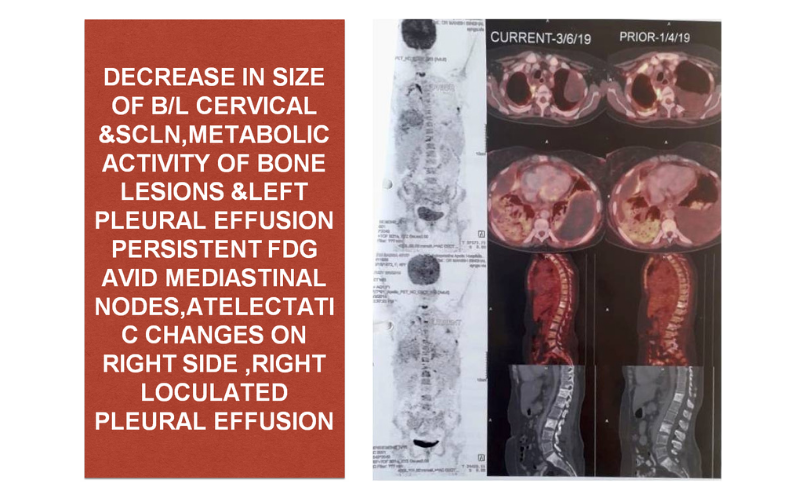
Treatment continues
-
Patient was started on weekly paclitaxel and bevacizumab, completed 1st cycle
-
Report of repeated NGS s/o BRAF V600E mutation and SMAD4 mutation positive
-
Started on DABRAFENIB 150 mg BD & TRAMETINIB 2mg OD ( since July 2019) on compassionate basis
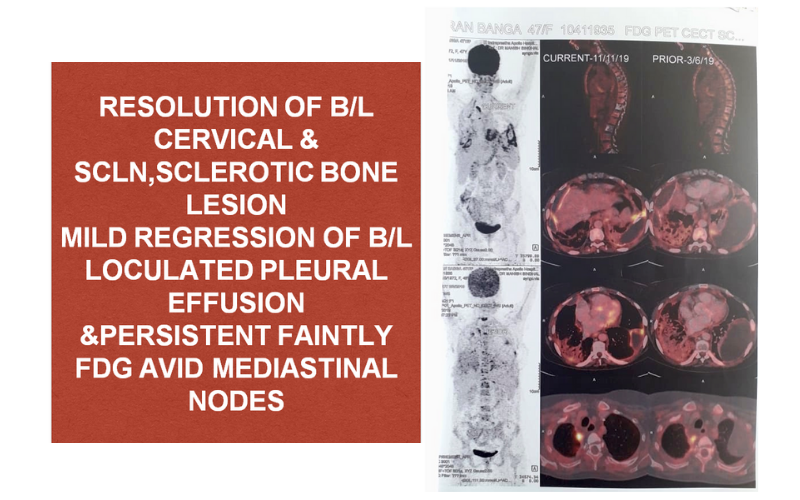
PRESENTLY: As of now patient is tolerating the combination of BRAF inhibitor and MEK inhibitor well and she had her last evaluation in May 2020 which was s/o stable disease.
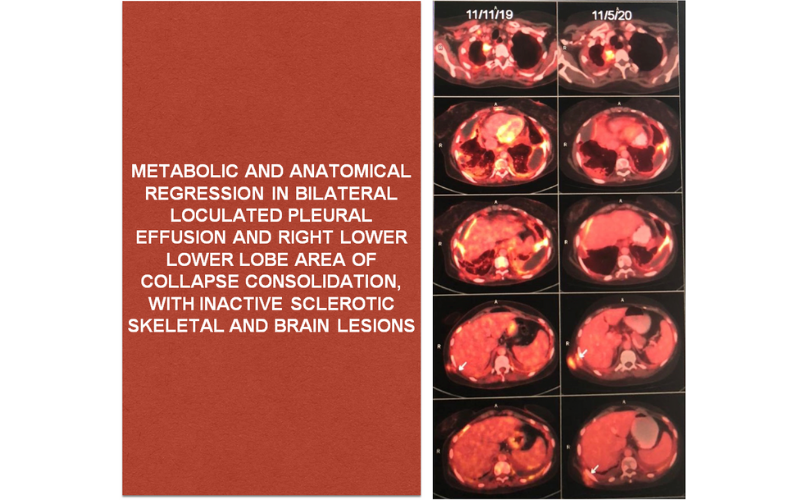
BRAF positive NSCLC
-
BRAF mutations have been documented in only 3.5-5% of the non-small cell lung cancer (NSCLC) patients (1)
-
The occurrence of BRAF V600E mutations account for 50% of these cases, and the rest of BRAF mutations are non-V600E
-
It is an oncogene that is a part of the RAS/MPK pathway that regulates cell growth and division
*(1) Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. Marchetti A, Felicioni L, Malatesta S, et al.*
-
BRAF- V600E mutations occur predominantly in females, smokers, and also has a pathology of adenocarcinoma (1,2)
-
On basis of phase ll study (Planchard et al) in previously treated advanced NSCLC with BRAF V600E mutation DABRAFENIB & TRAMETINIB had ORR of 63 % & DCR OF 79 % & mPFS of 9.7 months
*(2) Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. Litvak AM, Paik PK, Woo KM, et al.*




